Cellular Effects Following Exposure to WirelessDECT Base Radiation and Presentation of a Device for Their Compensation
Peter C. Dartsch1* and Timo Dochow2
1Dartsch Scientific GmbH, Institut für Zellbiologische Testsysteme, Auf der Voßhardt 25,
D-49419 Wagenfeld, Germany.
2IfFP Privates Institut für Feinstoffliche Forschung und Produktentwicklung GmbH, Oberaustraße 6b,
D-83026 Rosenheim, Germany.
Authors’ contributions
This work was carried out in collaboration between both authors. Author PCD designed the study,
performed the experiments and wrote the first draft of the manuscript. Author TD wrote the chapter
describing the operation principle of the compensation device. Both authors read and approved the
final manuscript.
The Impact of DECT Base Radiation on Cell Health and a Potential Solution
Introduction
The increasing prevalence of wireless communication devices, such as mobile phones, DECT phones, and routers, has led to a significant rise in environmental electromagnetic radiation. While the energy of this radiation is lower than ionizing radiation, research suggests it can affect biological processes, potentially leading to oxidative stress, cell death, and even carcinogenesis.
This study investigates the effects of radiation emitted from an active DECT base on cultured connective tissue cells and explores the potential of a newly created device to mitigate these effects.
Methods
An active DECT phone base was used to expose cultured connective tissue cells to radiation. A memonizerCOMBI Standard A device, utilizing specific mineral groups believed to counteract the negative effects of non-ionizing radiation, was tested for its ability to compensate for the DECT base radiation.
Cell vitality was assessed through morphological observation and enzymatic activity measurement.
Results
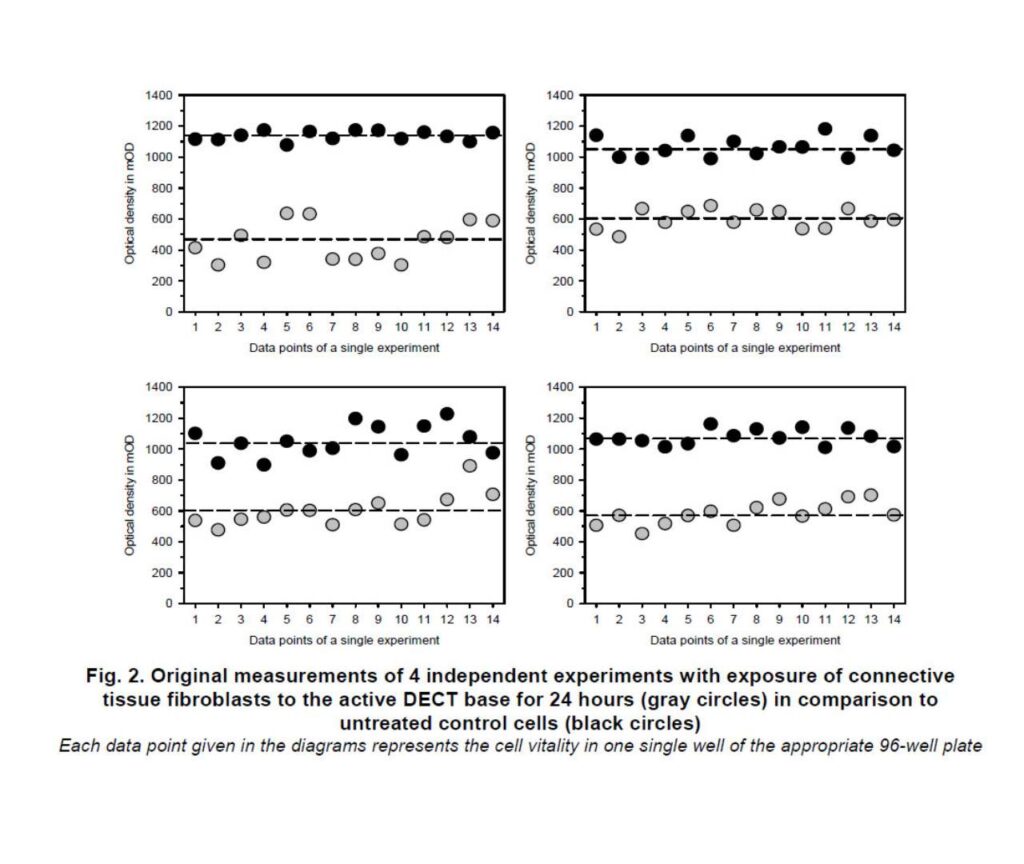
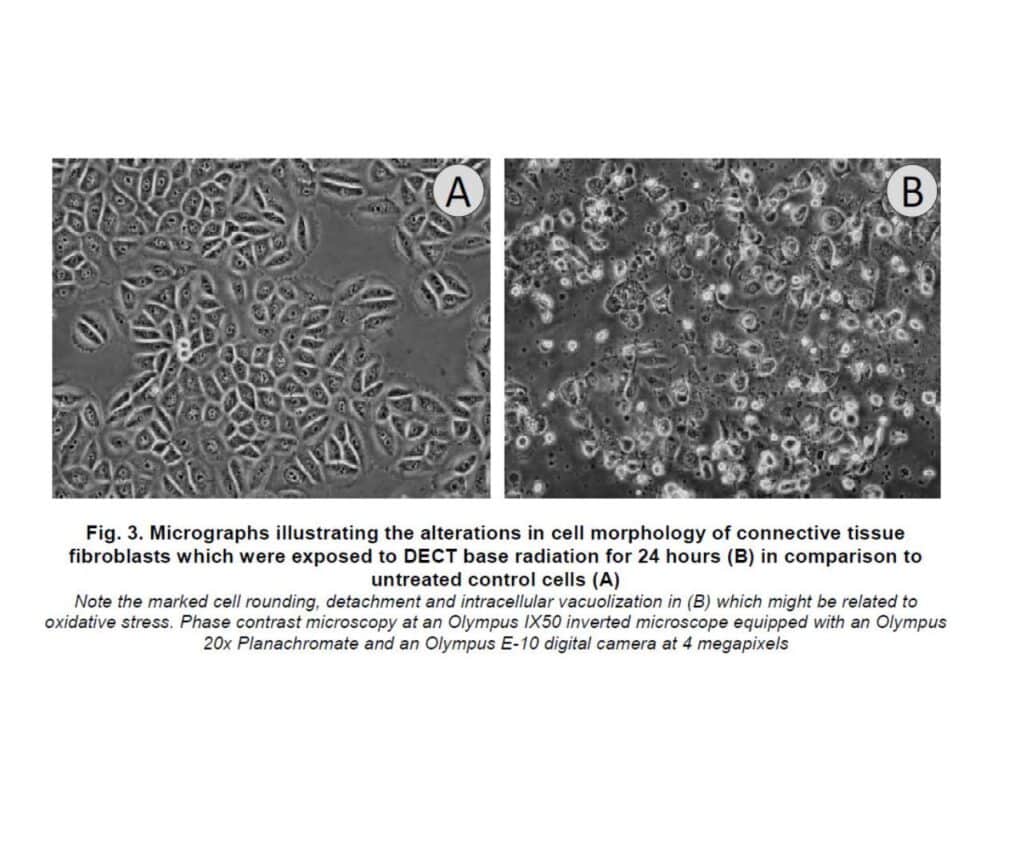
Exposure to the active DECT base for 24 hours resulted in a significant reduction in cell vitality and altered cell morphology. However, when the memonizerCOMBI device was introduced, the negative effects were reduced by approximately two-thirds.
Discussion
The findings highlight the potential health impact of DECT base radiation, even at distances typically encountered in daily life. The observed cellular effects are consistent with previous research suggesting that oxidative stress may be a key mechanism through which electromagnetic radiation affects cells.
The study demonstrates the ability of the memonizerCOMBI device to significantly counteract the negative cellular effects of DECT base radiation. The exact mechanisms behind this compensation warrant further investigation.
Conclusion
This study provides evidence that DECT base radiation can significantly impact cell health, and it introduces a promising solution for mitigating these effects. Further research is needed to fully understand the mechanisms involved and to explore the potential benefits of the memonizerCOMBI device for protecting against the broader range of electromagnetic radiation sources we encounter in our daily lives.
COMPETING INTERESTS
Authors have declared that no competing
interests exist.
REFERENCES
- Ahlbom A, Feychting M. Electromagnetic radiation: Environmental pollution andhealth. Br Med Bull. 2003;68:157-165.
- Havas M. Electromagnetic hypersensitivity: Biological effects of dirty electricity with emphasis on diabetes and multiple sclerosis. Electromagnetic Biol Med. 2006; 25:259-268.
- Levitt BB, Lai H. Biological effects from exposure to electromagnetic radiation emitted by cell tower base stations and other antenna arrays. Environ Rev. 2013;
18:369-395. - Margaritis LH, Manta AK, Kokkaliaris KD, Schiza D, Alimisis K, Barkas G, Georgiou E, Giannakopoulou O, Kollia I, Kontogianni G, Kourouzidou A, Myari A, Roumelioti F, Skouroliakou A, Sykioti V, Varda G, Xenos K, Ziomas K. Drosophila oogenesis as a bio-marker responding to EMFsources. Electromagn Biol Med. 2014; 33:165-189.
- Blank M and Goodman R. Electromagnetic fields stress living cells. Pathophysiology. 2009;16:71-78.
- Fragopoulou AF, Miltiadous P, Stamatakis A, Stylianopoulou F, Koussoulakos SL, Margaritis LH. Whole body exposure with GSM 900 MHz affects spatial memory in mice. Pathophysiology. 2010;17:179-187.
- Fragopoulou AF, Samara A, Antonelou MH, Xanthopoulou A, Papadopoulou A, Vougas K, Koutsogiannopoulou E, Anastasiadou E, Stravopodis DJ, Tsangaris GT, Margaritis LH. Brain proteome response following whole body exposure of mice to mobile phone or wireless DECT base radiation.Electromagn Biol Med. 2012;31:250-274.
- Sonmez OF, Odaci E, Bas O, Kaplan S.Purkinje cell number decreases in theadult female rat cerebellum followingxposure to 900 MHz electromagnetic
field. Brain Res. 2010;1356:95-101. - Kesari KK, Siddiqui MH, Meena R, VermaHN, Kumar S. Cell phone radiation exposure on brain and associated biological systems. Indian J Exp Biol.
2013;51:187-200. - Manta AK, Stravopodis DJ, Papassideri IS, Margaritis LH. Reactive oxygen species elevation and recovery in Drosophila bodies and ovaries following short-term and long-term exposure to DECT base EMF. Electromagn Biol Med. 2014;33:118-131.
- Vijayalaxmi, Scarfi MR. Biological health effects of radiofrequency fields. Int J Environ Res Public Health. 2014;11:9376-9408.
- Davis DL, Kesari S, Soskolne CL, Miller AB, Stein Y. Swedish review strengthens grounds for concluding that radiation from cellular and cordless phones is a probable human carcinogen. Pathophysiology. 2013;20:123-129.
- Hardell L, Carlberg M. Mobile phones, cordless phones and the risk for brain tumours. Int J Oncol. 2009;35:5-17.
- Chavdoula ED, Panagopoulos DJ, Margaritis LH. Comparison of biological effects between continuous and intermittent exposure to GSM-900-MHz mobile phone radiation: Detection of apoptotic cell-death features. Mutat Res. 2010;700:51-61.
- Fragopoulou AF, Koussoulakos SL, Margaritis LH. Cranial and postcranial skeletal variations induced in mouse embryos by mobile phone radiation. Pathophysiology. 2010;17:169-177.
- Panagopoulos DJ, Johansson O, Carlo GL. Real versus simulated mobile phone exposures in experimental studies. BioMed Res. 2016;Int Article ID 607053.
- Redmayne M, Inyang I, Dimitriadis C, Benkeab G, Abramson MJ. Cordless telephone use: Implications for mobile phone research. J Environ Monit. 2010;
12:809-812. - Liboff AR. Ion cyclotron resonance interactions in living systems. Società italiana biofisica elettrodinamica. Atti iv Convegno Nazionale Pavia. October 19,
2013. - Pitkänen M. More precise TGD based view about quantum biology and prebiotic evolution; 2015. Available:http://tgdtheory.com
- Ferris JP. Mineral catalysis and prebiotic synthesis: Montmorillonite-catalyzed formation of RNA. Elements. 2005;1:145-149.
- Sposito GS, Skippe NT, Sutton R, Park S, Soper AK, Greathouse JA. Surface geochemistry of the clay minerals. Proc Natl Acad Sci USA. 1999;96:3358–3364.
- Geesink JH, Meijer KF. Quantum wave information of life revealed: An algorithmfor electromagnetic frequencies that create stability of biological order, with
implications for brain function and consciousness. Neuro Quantology. 2016;14:106-125. - Avakyan SV, Voronin NA, Serova AE. The role of Rydberg atoms and molecules in the upper atmosphere. Geomag Aéronom. 1997; 37.
- Litovitz TA, Farrell JM, Barber M, Krause D. The superposition of a temporally incoherent magnetic field inhibits 60 Hzinduced changes in the ODC activity of
developing chick embryos. Bioelectromagnetics. 1998;19:53-56. - Hashizume H. Role of clay minerals in chemical evolution and the origins of life. 2012.DOI: 10.5772/50172
- Roehm NW, Rodgers GH, Hatfield SM, Glasebrook AL. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J
lmmunol Meth. 1991;142:257-265. - Brosin A, Wolf V, Mattheus A, Heise H. Use of XTT-assay to assess the cytotoxicity of different surfactants and metal salts in human keratinocytes (HaCaT). A feasible method for in vitro testing of skin irritants. Acta Dermatovenereologica. 1997;77:26-28.
- Lixia S, Yao K, Kaijun W, Deqiang L, Huajun H, Xiangwei G, Baohong W, Wei Z, Jianling L, Wei W. Effects of 1.8 GHz radiofrequency field on DNA damage and
Dartsch and Dochow; JOCAMR, 3(2): 1-9, 2017; Article no.JOCAMR.338499 expression of heat shock protein 70 in human lens epithelial cells. Mutat Res. 2006;602:135–142. - Hou Q, Wang M, Wu S, Ma X, An G, Liu H, Xie F. Oxidative changes and apoptosis induced by 1800-MHz electromagnetic radiation in NIH/3T3 cells. Electromagn Biol Med. 2015;34:85-92.
- Lantow M, Lupke M, Frahm J, Mattsson MO, Kuster N, Simko M. ROS release and Hsp70 expression after exposure to 1800 MHz radiofrequency electromagnetic fields in primary human monocytes and lymphocytes. Radiat Environ Biophys. 2006; 45:55–62.
- Lantow M, Schuderer J, Hartwig C, Simko M. Free radical release and HSP70 expression in two human immune-relevant cell lines after exposure to 1800 MHz
radiofrequency radiation. Radiat Res. 2006;165:88–94. - Dasdag S, Akdag MZ. The link between radiofrequencies emitted from wireless technologies and oxidative stress. J Chem Neuroanat. 2016;75: 85-93.
Recent Posts
Top Products
-
EMF MemonizerCar
$592.00 – $810.00Price range: $592.00 through $810.00 -
EMF memonizerMOBILE
$149.00 -
EMF memonizerCOMBI plug
$1,350.00 – $3,950.00Price range: $1,350.00 through $3,950.00 -
EMF memonizerWATER
$1,150.00 -
EMF memonizerHEADSET
$149.00 -
EMF memonizerEARPHONE
$149.00 -
EMF memonizerFOOD
$140.00 -
EMF memonizerWLAN
$305.00 -
EMF memonizerBODY pendant
$405.00